US Food Safety Policy: New Labeling Rules Explained

The evolving landscape of US food safety policy, particularly with new labeling requirements, significantly impacts consumers, manufacturers, and regulators by enhancing transparency and preventing misrepresentation of food products.
The intricate world of food safety is constantly evolving, with new regulations and requirements emerging to protect consumer health and ensure fair practices. A significant area of focus recently has been the **US Food Safety Policy: Understanding the New Labeling Requirements and Their Impact**, a critical development shaping how food products are manufactured, marketed, and consumed. These updates are far from cosmetic; they represent a concerted effort to enhance transparency and provide consumers with clearer, more actionable information about the food they purchase.
The Evolution of US Food Safety Regulations
For decades, US food safety regulations have aimed to safeguard public health, prevent foodborne illnesses, and ensure fair trade practices. Historically, this involved setting standards for food processing, handling, and storage. However, as supply chains grew more complex and consumer awareness increased, the need for more granular information became evident.
The journey towards modern food labeling began with basic ingredient lists, evolving to include nutritional facts panels in the early 1990s. This marked a pivotal shift, granting consumers unprecedented access to dietary information. Yet, gaps remained, particularly concerning allergens, genetically modified organisms (GMOs), and newer dietary trends.
Key Milestones in Labeling History
Several legislative acts have incrementally shaped current labeling paradigms. Each step aimed to address emerging public health concerns or marketplace demands.
- Nutrition Labeling and Education Act (NLEA) of 1990: Mandated nutrition labels on most packaged foods, standardizing nutrient declarations and health claims.
- Food Allergen Labeling and Consumer Protection Act (FALCPA) of 2004: Required clear labeling of major food allergens, a crucial step for millions with severe allergies.
- Bioengineered Food Disclosure Standard (National Bioengineered Food Disclosure Standard) of 2018: Established a national mandatory standard for disclosing foods that are bioengineered (GMOs), aiming for transparency.
These foundational policies laid the groundwork for the most recent updates, reflecting a continuous adaptation to scientific advancements and consumer expectations. The underlying principle has always been empowerment: providing consumers with the knowledge to make informed decisions about their food choices.
The ongoing evolution signifies a dynamic regulatory environment, constantly striving for a balance between industry feasibility and consumer protection. Each new requirement builds on the last, creating a comprehensive framework for food transparency.
Understanding the Core Changes in New Labeling Requirements
The latest wave of labeling requirements touches on several critical areas, moving beyond basic nutritional information to address emerging consumer concerns and scientific understanding. These changes reflect a proactive approach by regulatory bodies to enhance clarity and accuracy on food packaging.
At the heart of these updates is an enhanced emphasis on critical nutritional components, allergen declarations, and specific disclosures related to food production methods. The goal is to make labels more intuitive and informative for the average consumer, moving away from overly technical jargon.
Key Areas of Update
The revisions are broad, but several stand out due to their significant impact on both manufacturers and consumers:
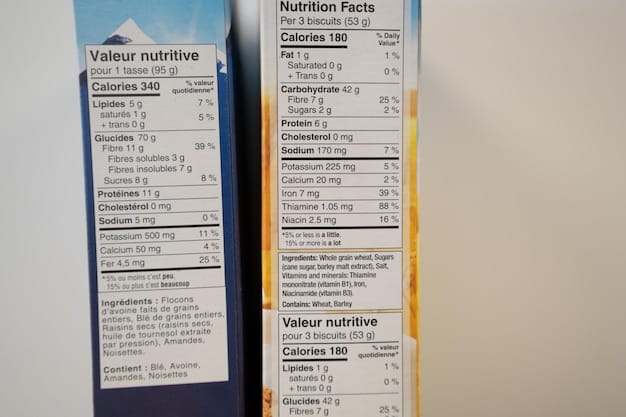
- Updated Nutrition Facts Panel: This is perhaps the most visible change, featuring a refreshed design, larger calorie and serving size declarations, and updated daily values for various nutrients. It also includes “Added Sugars,” a new mandatory declaration.
- Allergen Declaration Enhancements: While FALCPA laid the groundwork, recent efforts aim for even greater clarity and specificity regarding allergen cross-contact and advisory statements, particularly concerning sesame, which was recently added as a major allergen.
- Bioengineered Food Disclosure: Implementation of the Bioengineered Food Disclosure Standard means products containing detectable bioengineered DNA must bear a symbol, text, or electronic link (QR code) indicating their “bioengineered” status.
These updates are designed to be more user-friendly, with a focus on quick comprehension. For instance, the redesign of the Nutrition Facts Panel stems from research on how consumers actually read and interpret labels, aiming to highlight the most crucial information upfront.
Beyond these, there’s growing scrutiny on marketing claims, with regulators evaluating terms like “natural” or “healthy” to ensure they are not misleading. This holistic approach ensures that the entire label, not just specific sections, conveys accurate and truthful information.
Impact on Food Manufacturers and Supply Chains
The implementation of new labeling requirements places significant responsibilities on food manufacturers, extending beyond just reprinting packaging. It necessitates a comprehensive review of product formulations, supply chain tracing, and internal documentation procedures.
Compliance often involves substantial investment in research and development, particularly for smaller businesses that may lack dedicated regulatory compliance teams. Manufacturers must rigorously analyze their ingredients and production processes to ensure every claim on the label can be substantiated.
Challenges and Opportunities for Businesses
The transition period for new labeling standards can present a steep learning curve. However, it also offers opportunities for innovation and building stronger consumer trust.
- Cost of Compliance: Updating packaging designs, recalibrating nutritional analysis, and tracing supply chain ingredients to verify claims can be costly. This often includes redesigning thousands of product SKUs.
- Data Management Complexity: Managing accurate data for a multitude of ingredients and finished products across diverse supply origins becomes paramount. Robust data systems are crucial for maintaining compliance.
- Consumer Trust and Market Advantage: Companies that proactively embrace transparency and clearly communicate label changes can gain a competitive edge by fostering greater consumer trust and loyalty.
Furthermore, these regulations compel companies to reassess their ingredient sourcing. For instance, the focus on added sugars might encourage manufacturers to reformulate products to reduce sugar content, leading to healthier alternatives and potentially new product lines.
Integrating these changes effectively into existing supply chains also requires enhanced collaboration with suppliers and distributors. Ensuring all parties are aware of and adhere to the new standards is critical to avoid costly mistakes or recalls.
Consequences for Consumers and Public Health
The ultimate beneficiaries of enhanced food labeling are consumers and public health initiatives. Clear, accurate labels empower individuals to make more informed dietary choices, contributing to better health outcomes and a reduction in diet-related illnesses.
By providing transparent information, these policies help consumers manage conditions like allergies, diabetes, and heart disease more effectively. The reduction of misleading information also promotes a healthier relationship between consumers and the food industry.
Empowering Informed Food Choices
The direct impact on consumer behavior is multifaceted:
- Improved Nutritional Awareness: The updated Nutrition Facts panel, with its focus on “Added Sugars” and clear serving sizes, educates consumers about what they are truly consuming, aiding in weight management and chronic disease prevention.
- Enhanced Allergen Safety: More precise allergen declarations significantly reduce the risk of accidental exposure for individuals with severe food allergies, providing a crucial layer of safety.
- Transparency Regarding Production: Disclosure of bioengineered ingredients offers consumers the choice to align their purchases with their personal beliefs and values regarding food production methods.
Beyond individual choices, the aggregated impact on public health is substantial. For example, a widespread reduction in “added sugars” across packaged goods could lead to a measurable decrease in obesity and type 2 diabetes rates over time.
The labeling requirements also strengthen the relationship between regulators and consumers, reinforcing the perception that government agencies are actively working to protect public interest in the food supply.
Challenges in Enforcement and Compliance
While the intent behind new labeling requirements is clear, the path to universal enforcement and compliance is often paved with challenges. Regulatory bodies face the complex task of monitoring thousands of food producers and ensuring adherence across a vast and diverse marketplace.
The sheer volume of products and manufacturers, coupled with evolving scientific understanding and industry innovation, creates a dynamic environment. Furthermore, inconsistencies can arise, particularly with imported goods or products from smaller, niche producers.
Overcoming Regulatory Hurdles
Effective enforcement requires a multi-pronged approach and continuous adaptation:
- Resource Allocation: Regulatory agencies, such as the FDA and USDA, require adequate funding and staffing to conduct inspections, review labels, and pursue non-compliant entities.
- Education and Outreach: Proactive efforts to educate manufacturers, especially small businesses, about new requirements can significantly improve compliance rates and reduce unintentional violations.
- Technological Solutions: Utilizing data analytics and AI to identify potential non-compliance patterns or to streamline the label review process can enhance enforcement efficiency.
One significant challenge is harmonizing international standards, especially for products intended for global markets. Discrepancies between different countries’ labeling laws can complicate compliance for multinational corporations.
Moreover, the ongoing debate around the interpretation of certain terms, like “natural,” means regulators must continuously refine guidelines and engage with stakeholders to achieve clarity and consensus.

Future Outlook: Upcoming Trends in Food Labeling
The current labeling changes are not the end of the journey but rather a stepping stone in the continuous evolution of food safety policy. Several emerging trends suggest that future labeling requirements will delve even deeper into areas of sustainability, ethical sourcing, and personalized nutrition.
The confluence of consumer demand for greater transparency, technological advancements, and a growing understanding of the environmental and social impact of food production is driving these anticipated changes.
Anticipated Areas of Focus
Looking ahead, we can anticipate several key themes influencing future labeling policies:
- Sustainability and Environmental Impact: Labels might begin to include information on a product’s carbon footprint, water usage, or packaging recyclability, mirroring a broader societal shift towards environmental consciousness.
- Ethical Sourcing and Animal Welfare: With increasing consumer concern for how food is produced, labels could include certifications related to labor practices, fair trade, or animal welfare standards (e.g., “pasture-raised,” “cage-free”).
- Personalized Nutrition and Health Claims: As our understanding of individual dietary needs grows, there may be a push for more dynamic or personalized labeling, possibly through scannable codes that link to detailed, customized nutritional advice.
The rise of digital solutions, such as QR codes linking to comprehensive product databases, offers a promising avenue for conveying vast amounts of information without cluttering physical labels. This could enable real-time updates and more detailed disclosures than current static labels.
Furthermore, the integration of blockchain technology could revolutionize supply chain transparency, providing an immutable record from farm to fork, which could then be reflected on future labels to verify origin and production methods.
| Key Aspect | Brief Description |
|---|---|
| 📊 New Nutrition Panel | Features larger calories, serving sizes, and mandatory “Added Sugars.” |
| ⚠️ Allergen Clarity | Enhanced specificity for major allergens, including sesame. |
| 🌱 Bioengineered Disclosure | Mandatory labeling for foods with detectable bioengineered DNA. |
| 💰 Manufacturer Impact | Significant costs for redesigns, data management; opportunities for trust. |
Frequently Asked Questions About US Food Labeling
“Added Sugars” refers to sugars and syrups put into foods during processing, or packaged as such, not those naturally occurring in foods like fruit or milk. This new category helps consumers distinguish between natural and supplemental sugar content for better dietary control.
Small businesses often face greater challenges due to limited resources for redesigning labels and updating nutritional analyses. However, regulatory bodies often provide extended compliance deadlines or specific guidance to help them adapt to the new mandates, ensuring smoother transitions.
Bioengineered food refers to products containing detectable genetic material that has been modified through certain lab techniques and cannot be created through traditional breeding. It is labeled with a “bioengineered” symbol, text, or a QR code linking to disclosure details, as per the 2018 standard.
Yes, during transition periods, it’s common to find both old and new labels on store shelves as manufacturers deplete old packaging. However, eventually, all products must comply with the updated requirements. The phased approach helps reduce waste and gives companies time to adapt.
Sesame was added as the ninth major food allergen due to a significant increase in reported allergic reactions and its widespread use in numerous food products. This classification requires it to be clearly declared on food labels, improving safety for individuals with sesame allergies under FALCPA.
Conclusion
The evolving landscape of US food safety policy, particularly with the new labeling requirements, represents a significant step forward in consumer protection and market transparency. These regulations empower consumers with clearer, more comprehensive information, fostering healthier dietary choices and enhancing safety for those with allergies. While posing challenges for manufacturers in terms of compliance and cost, the long-term benefits of greater trust and informed decision-making are undeniable. As technology advances and consumer demands shift, food labeling will undoubtedly continue its evolution, moving towards even greater detail on sustainability, ethical sourcing, and personalized nutrition, cementing its role as a cornerstone of public health.